A testing laboratory, ADIP TRLab Inc adheres to the standards for chemical and microbial analyses and state-of-the-art equipment to guarantee that the results of analyses are attested.

Stability Storage and/or Stability Testing
Provide stability storage and/or stability testing for initial product registration as per ASEAN Guideline on the Stability Study of the Drug Product and on-going stability requirement and as per PIC/S Guide to GMP for Medicinal Products Part I. Also performs stability testing and storage for cosmetics, traditional medicines and food supplements as per customer requirements.
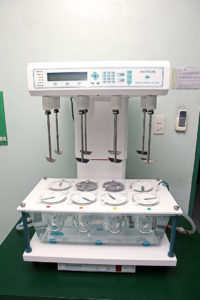
Dissolution Profile
Our lab is capable of performing dissolution tests and dissolution profiles as required by the regulatory agency using the medium/media as per WHO and Philippine FDA Standards and official reference monographs.


Elemental Impurities / Heavy Metals Testing using Inductively Coupled Plasma - Optical Emission Spectrometry (ICP-OES)
Capable of testing elemental impurities for drug products as per latest compendia using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) including the determination of heavy metals for traditional medicines, cosmetics and veterinary products and materials.
High Performance Liquid Chromatography (HPLC)
Identify an analyte or an active component in a given product as well as identify an unknown material against a known standard (qualitative analysis). This can also be used to determine the amount of the analyte/active component in a material or the amount of one or more active materials in a product (quantitative analysis). Impurity testing can also be performed using HPLC.



Identification by Fourier Transform Infrared (FTIR)
Each material has a unique infrared fingerprint that can be used to identify them in comparison with a reference standard. Our FTIR equipment can determine if two materials are, in fact, the same. This can also confirm if the active ingredient in some finished drug products are truly present.
Identification by Thin Layer Chromatography (TLC)
Determine the identification of a material and the active ingredient of a product through TLC as per the monograph in the official compendia or any available reference.

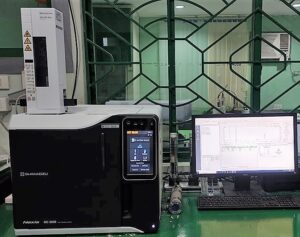
Gas Chromatography (GC)
Also used for the quantitative and qualitative testing of materials and products but more specifically used for samples that are volatile or can be converted into volatile samples.
Water Determination by Karl Fischer (KF)
Measure the water content of materials and products through the Karl Fisher apparatus, one of the most commonly used titrimetric procedure to determine the amount of water in a substance.


UV/Vis Spectrophotometer
A more conventional way of performing the qualitative and quantitative analysis of an unknown sample.
Microbiological Testing
Raw materials and products microbiological testing capabilities performed as per latest compendial and registration requirements. Also provide environmental testing as required by the customers.












